Platform Introduction
Liwen provides one-stop oncology precision medicine solutions to support drug discovery, clinical trial marker testing and concomitant diagnosis for pharmaceutical companies and research users. Liwen has a central laboratory accredited by the College of American Pathologists (CAP) and a multi-level technology platform covering molecular, cellular and animal levels including second-generation sequencing, fluorescence in situ hybridization, fluorescence quantitative PCR, immunohistochemistry, immunofluorescence, circulating tumor cells, enzyme-linked immunoassay, PDX, MiniPDX, etc. The testing and management of samples are strictly supervised in CAP's quality system through LIMS and BIMS under the system. For sample collection and transportation, Liwen has its own sampling cassette system to ensure the quality control of sampling and transportation process for various sample types.
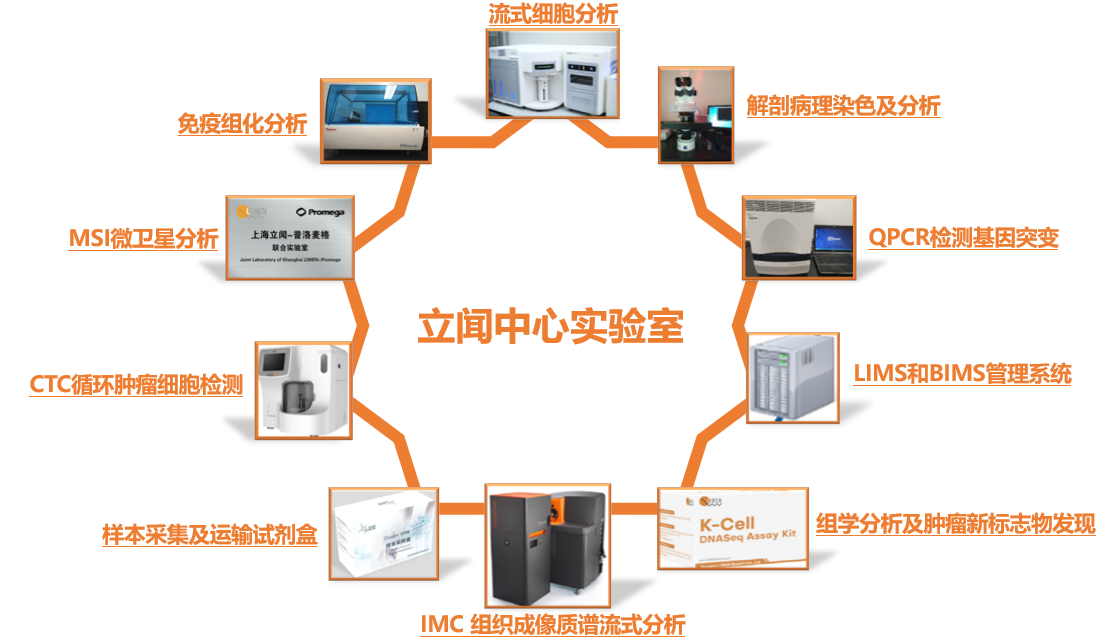
Service Approach
Liwen has developed a wide range of marker tests, in addition to providing customized services.
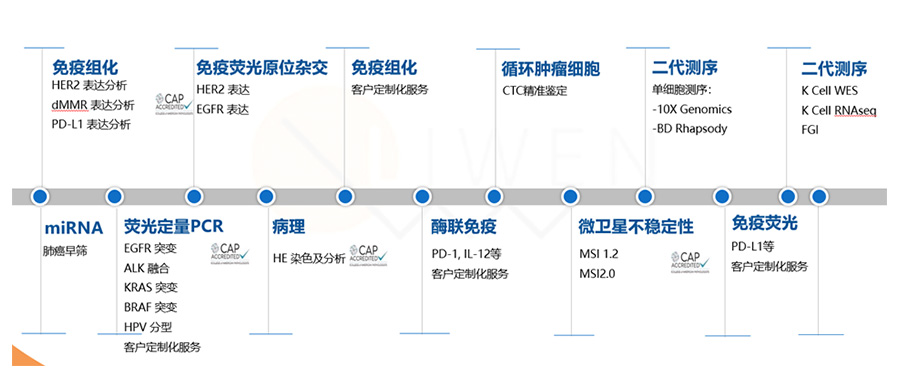
Technical Advantages
One-stop, multi-level technology platform
Full molecular, pathological and functional coverage.
Abundant sample resources
200+ partner hospitals and our own sample bank.
First-class quality system
CAP certification, LIMS & BIMS management.
First-class technical and management team
Extremely experienced pathologists and technologists.
Application Examples
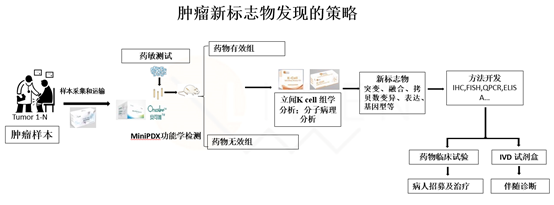
Example 1. New tumor marker discovery
FGFR is a long-discovered gene associated with tumorigenesis, with four isoforms and some heterodimeric molecules, and various types of changes, such as mutations, translocations, and expression changes, etc. Whether it is a tumor driver gene is unclear. Before the successful launch of FGFR2 inhibitor kinase inhibitor Pemigatinib, about 270 clinical trials targeting FGFR failed.
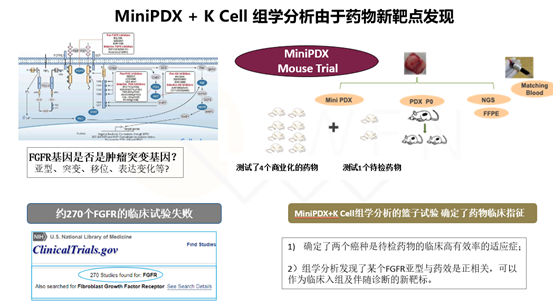
In this case, the drug to be tested was found to be the most effective drug in two tumors in the functional pharmacological assay using MiniPDX; further, in the histological analysis of the drug effective group and the drug ineffective group in these two tumors, it was found that a certain isoform of FGFR was positively correlated with the drug effect, which means that this FGFR isoform is a new marker of the drug effect in tumors; meanwhile, the two drug effective tumors, which are the indications of the drug in clinical trials.
By adopting Liwen's unique strategy of new tumor marker discovery, we can realize the marker discovery, mechanism study and indication selection of new drugs.
Example 2. IHC and FISH techniques for detection of tumor markers
Liwen's CAP lab offers multiple case monitoring including IHC in the mainland. IHC and FISH testing for HER2 in breast cancer is shown below.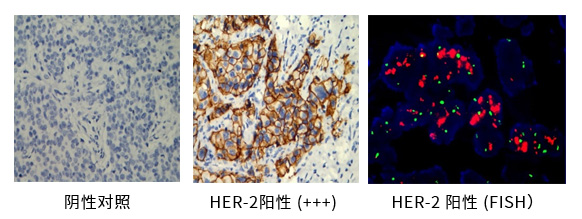
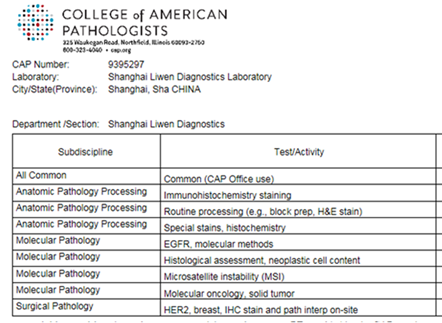
Liwen's CAP certification
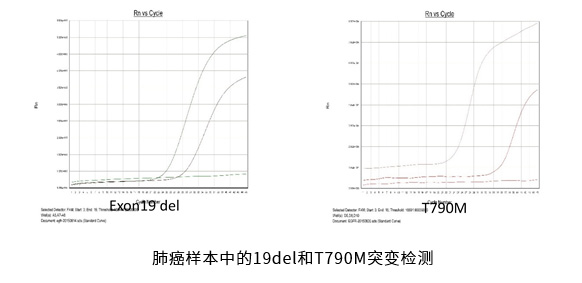
Example 3. QPCR technique for detection of tumor markers
Detection of EGFR mutations in lung cancer samples by QPCR.
Detection of ALK fusion mutations in lung cancer samples by QPCR.
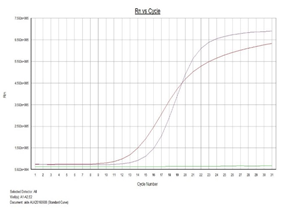
Detection of EML4-ALK fusion mutations in lung cancer samples.








